8 Comments
- Log in to leave a comment
-
4,058 Mikel8+2 3.5 years ago
Just a question... why are the carbon-carbon bonds on the benzene molecule purple ?
Shouldn't them be black as well ?
I love physics and chemicals but i never seen this.
Verry good post tho !
(Next time do all the ergols used in space discovery, classic propelants as well as hypergolics maybe.) -
-
-
-
-
-
15 Upvotes
Log in in to upvote this post.



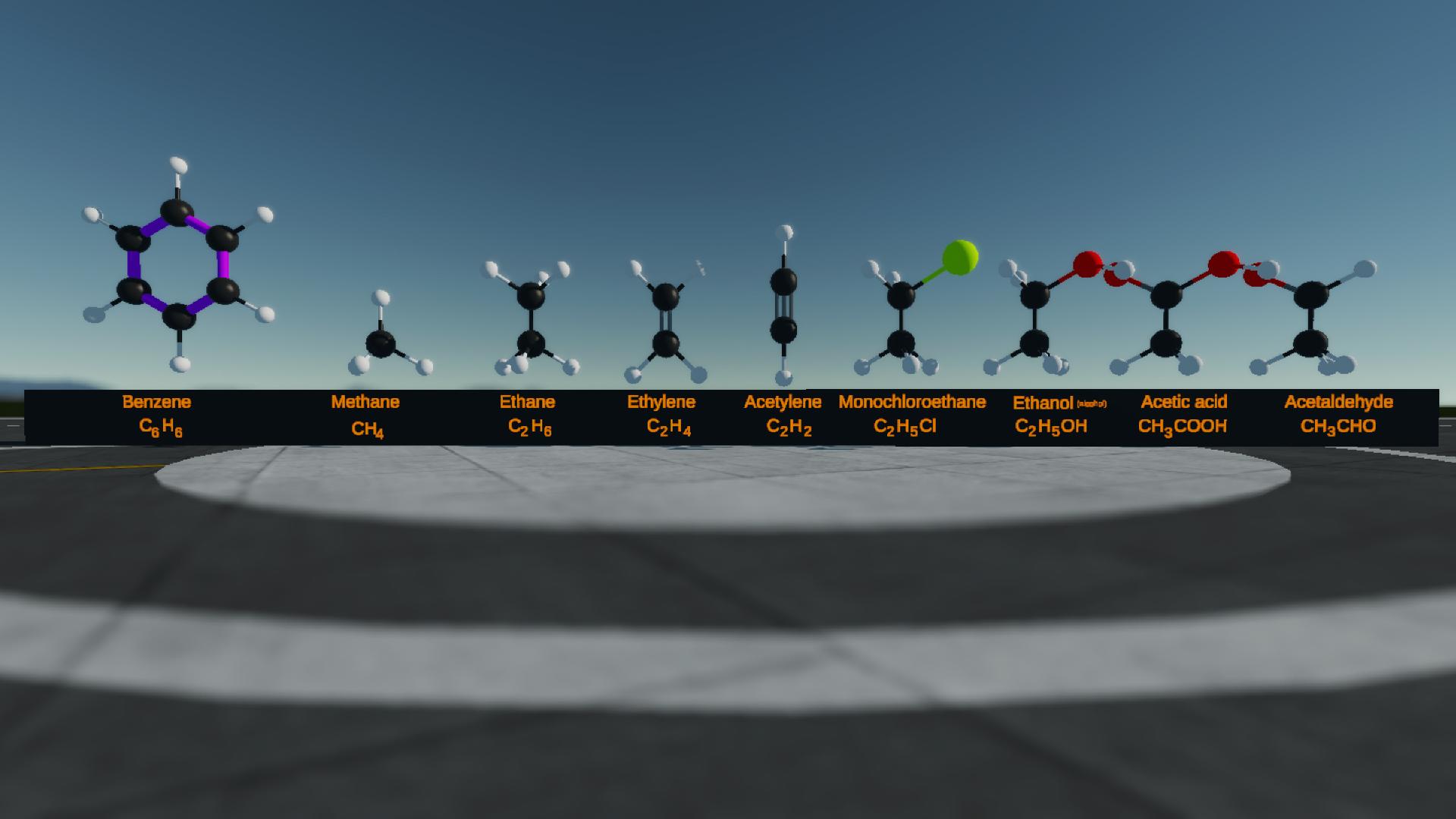
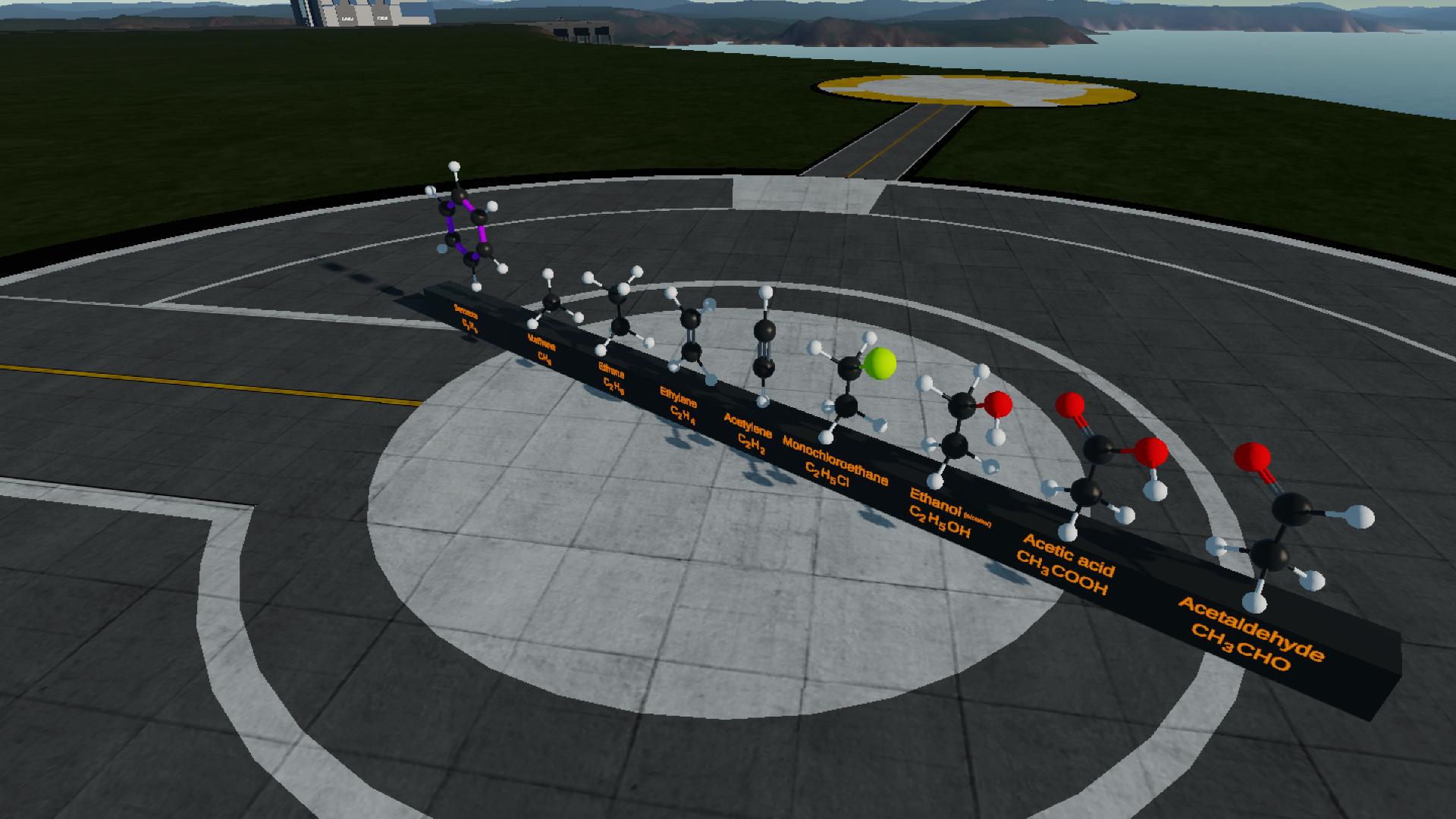
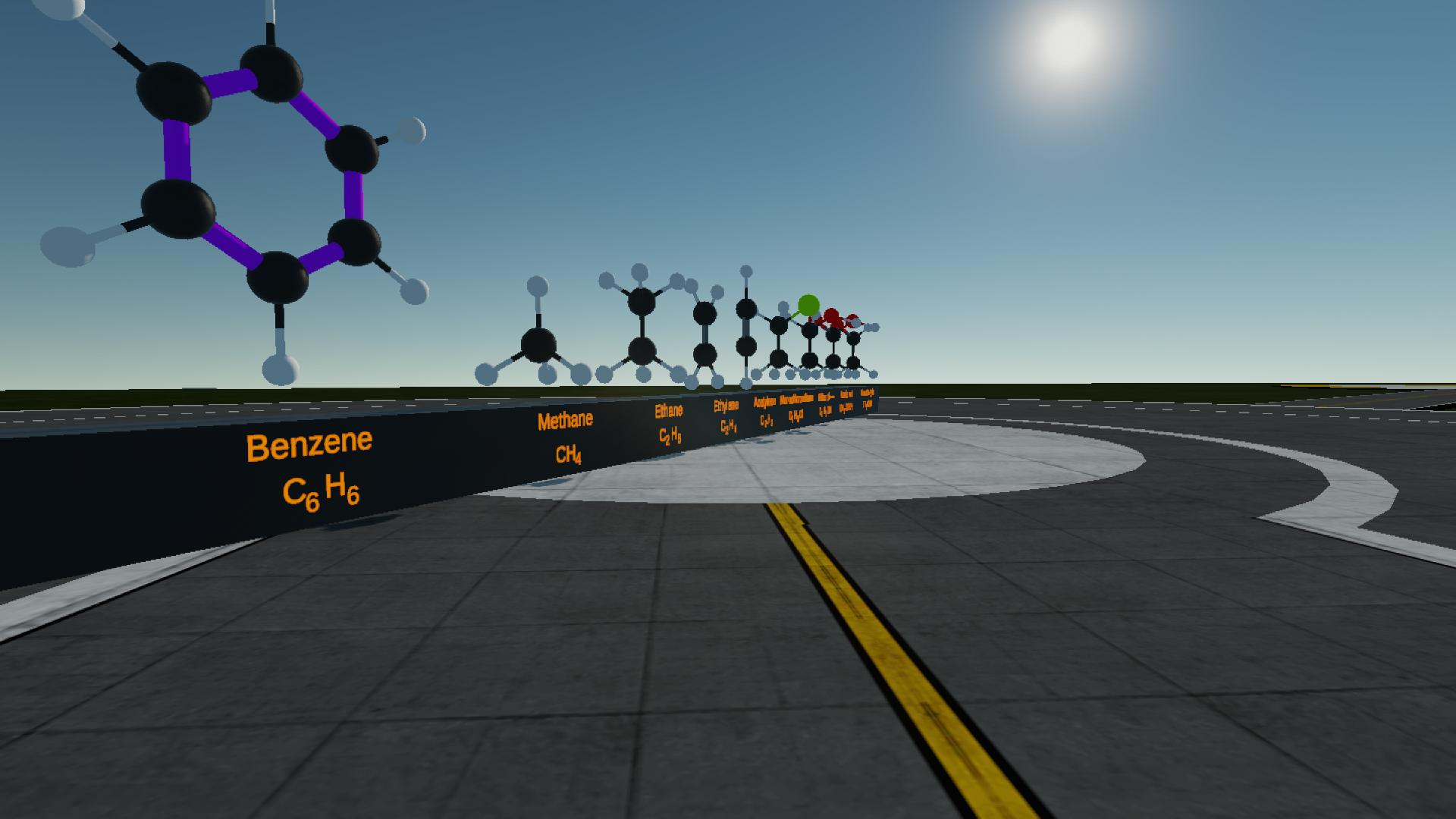
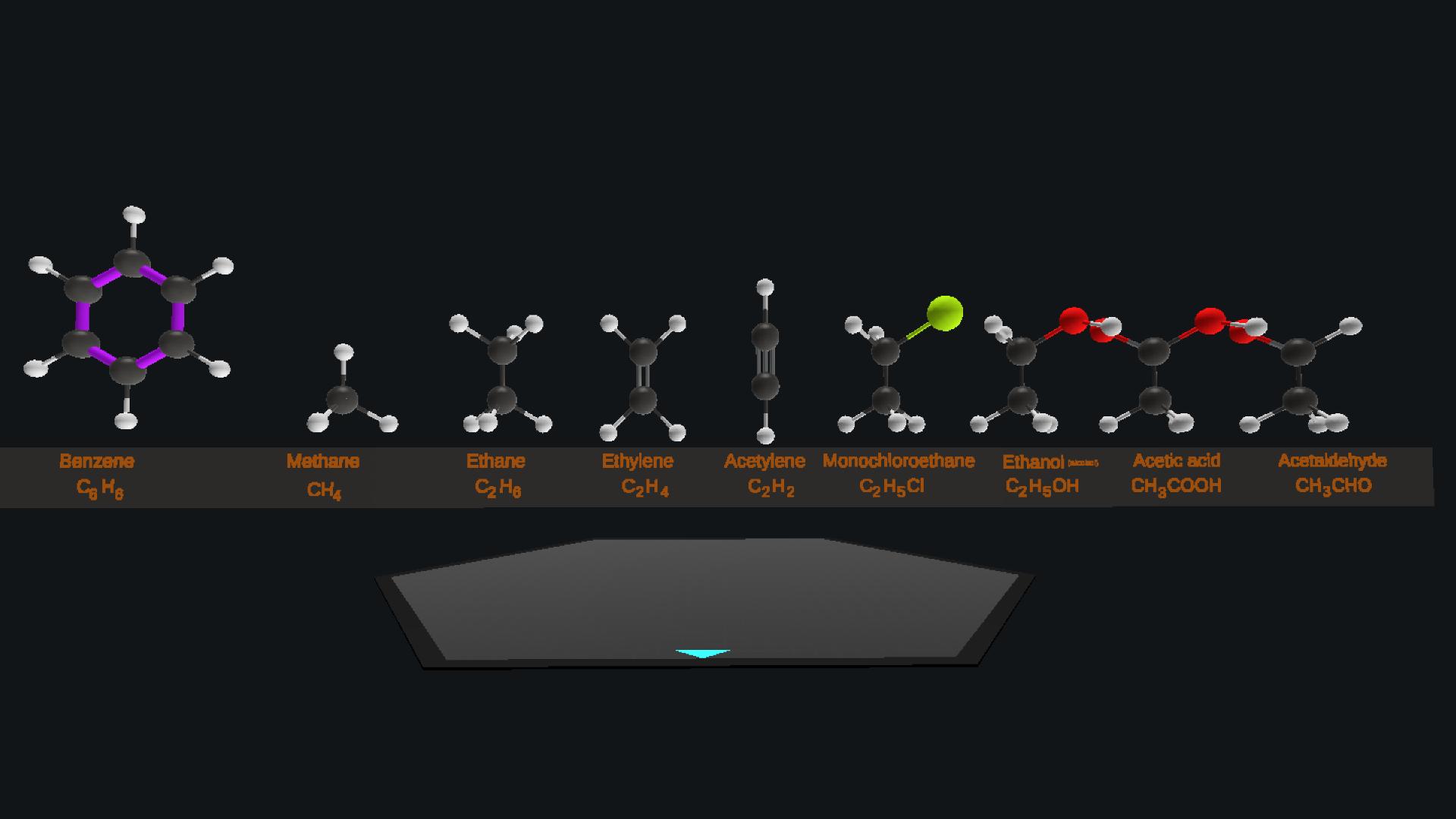
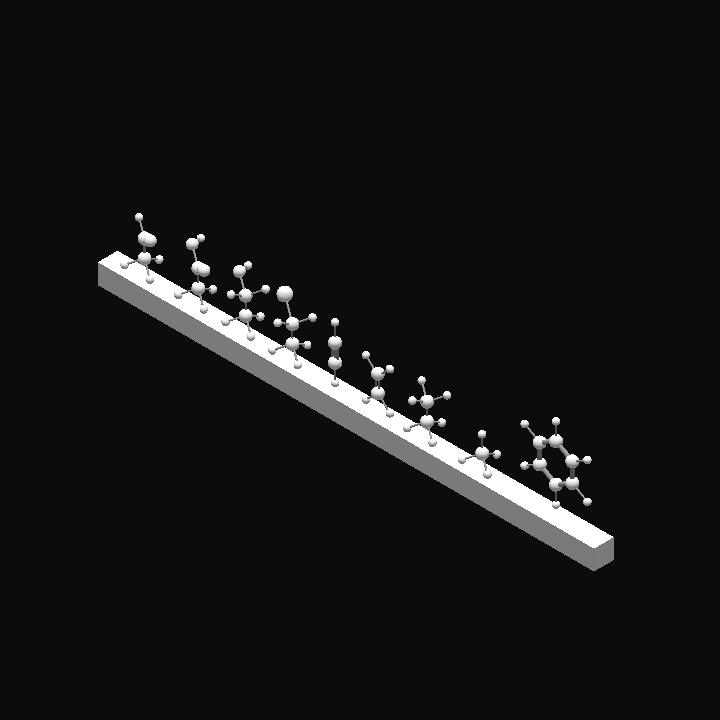

Well the carbon-carbon bond in benzene is different from other chemicals because it is a delocalized pi bond .So I just painted it with purple in order to distinguish it from others @Mikel8